Clinical data management
METRONOMIA, champions for better data
At METRONOMIA, we take pride in being champions for better data, ensuring that it is optimally defined, stored, monitored and processed for maximum value in your development portfolio.
Our people, processes and technology ensure that your data is:
- Of the highest quality and precision, meeting or surpassing requirements and gold standards
- Precisely monitored within a fluid feedback-loop allowing identification of risks/mitigations and opportunities
- Protected and secured in a capsulated environment
- Ready to pass when put to the test by regulatory agencies
- A commodity in your clinical development portfolio by achieving its full potential
With a full range of clinical data management services, METRONOMIA’S data managers are your experts for:
- Clinical studies of all sizes and all phases
- New/existing drugs and medical devices
- Stand-alone projects or complete clinical development programs
- Outsourced data management acting fully as your data management representatives or as an extension of your own data management team
Your Single Data Center collaborating in unison with our Biostatistical group. Allow us to share our success stories, made possible by unsurpassable agility and dedication! Contact us for an appointment or references!
Clinical data management services comprise
- Outsourced Clinical Data Project Managers & Data Quality Control Managers
- Clinical Development Portfolio, Program/Plan or Protocol consultation
- Vendor Management
- Technology Solutions Consulting
- Risk-Based Monitoring Strategies
- Trial Master File Management
Metronomia is providing for three leading EDC systems, CLINCASE, RAVE and VIEDOC, comprehensive services:
- eCRF Design & Development
- eCRF Annotation & Review
- Database Design and Validation
- Edit check programming, validation, and testing, UAT
- Electronic Clinical Outcome Assessment (eCOA) and Electronic Patient Reported Outcome (ePRO) Design, Development & Validation
- Set-up for targeted / selective SDV
- Custom reports
- Supporting 4 leading EDC systems: Medidata Rave, Veeva Vault, Viedoc, and Clincase
- eCRF access and user management
- Development of training material, eCRF user manuals and completion guidelines
- eCRF self-training, live-training and train–the-trainer
- SAE reporting in E2B format
- Integration with third-party eDiary/ePRO systems
- Integration with third-party CTMS systems
- 24/7 EDC helpdesk
- Import, reconciliation, and cleaning of all types of electronic data (lab, ECG, PK, eDiary, photo etc.)
- Patient diary and PRO data management
- Local lab data management
- Continuous data review & validation
- Data cleaning & query management
- Serious Adverse Event (SAE) Reconciliation
- Medical / Manual / SAS® Data Listings Review
- Medical coding (MedDRA, WHO-DD or client’s dictionary)
- Real-time data viewing and reporting
- Database snapshots, soft & hard lock
- Bespoke clinical data warehousing solutions
- Interactive data visualisation
- Cross study analytics
- Support for multiple EDC solutions and other data sources
- Data Management Plan
- Data Validation Plan
- CRF Completion Guidelines (CCG) Development
- Data archival and storage
- Data export & transfer
- CDISC CDASH and SDTM compliant deliverables
We ensure compliance with ICH-GCP E6 (R2) oversight obligations on your behalf for outsourced statistical and data management functions:
- Subject matter experts (SMEs) in stats and clinical data management provide oversight
- High quality review of documents and deliverables received from vendors
- Expert input to oversight management plan
- Risk-based oversight activities compliant with ICH-GCP E6 (R2)
- Support of vendor selection
- Tailored implementation of Stats and clinical data management relevant KPIs
Metronomia is supporting four leading EDC systems each offering fully integrated solutions for eCRF, ePRO/eCOA, targeted SDV, IRT, randomization and others:
In addition, seeWise supplements our data focused strategy with powerful data visualization and medical monitoring & review functionality.
read more about our non-clinical services.
- Processing of in vitro or in vivo raw data and large complex data sets in an evaluable and publishable form: tables, graphs, presentations
- Data visualization
- Processing data in SEND format
- Biological data management and set-up of databases for non-clinical data
- Systems and SOPs still available and maintained for high quality paper process
- Serving strong demand for hybrid studies – eCRF plus paper-PROs or mixed paper- and e-PROs
- Supporting paper CRFs and paper-based PROs
- Data processing through double data entry
- Independent verification – data entry QC
Supported EDC Systems
Selecting the right EDC system for your study is important. At METRONOMIA we support the following four leading EDC systems.
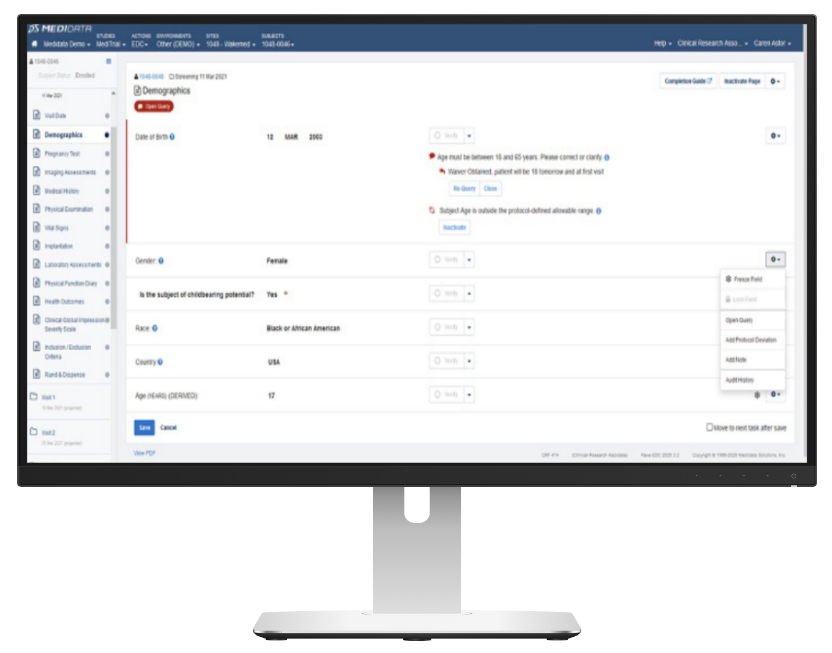
Medidata Rave
- Widely considered the EDC market leader
- Cornerstone of the Medidata Platform
- Mature and comprehensive system
- Very well accepted
Clincase
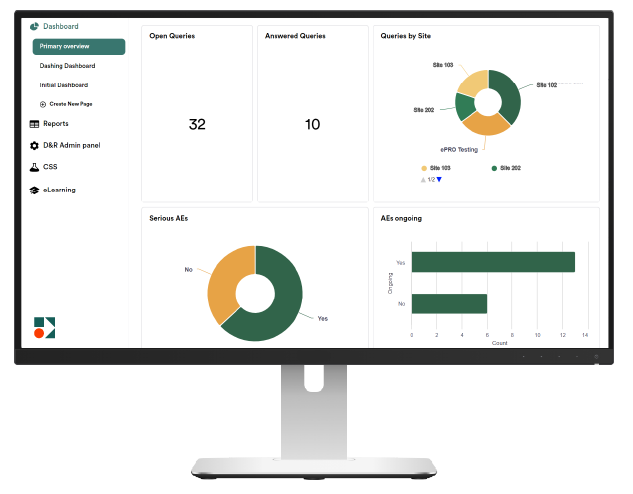
- User-Friendly, intuitive Interface
- Customizable, very flexible eCRFs
- Robust Data Security; full compliance with regulatory standards
- Fully Integrated Modules: eCRF, ePRO, RTSM, coding
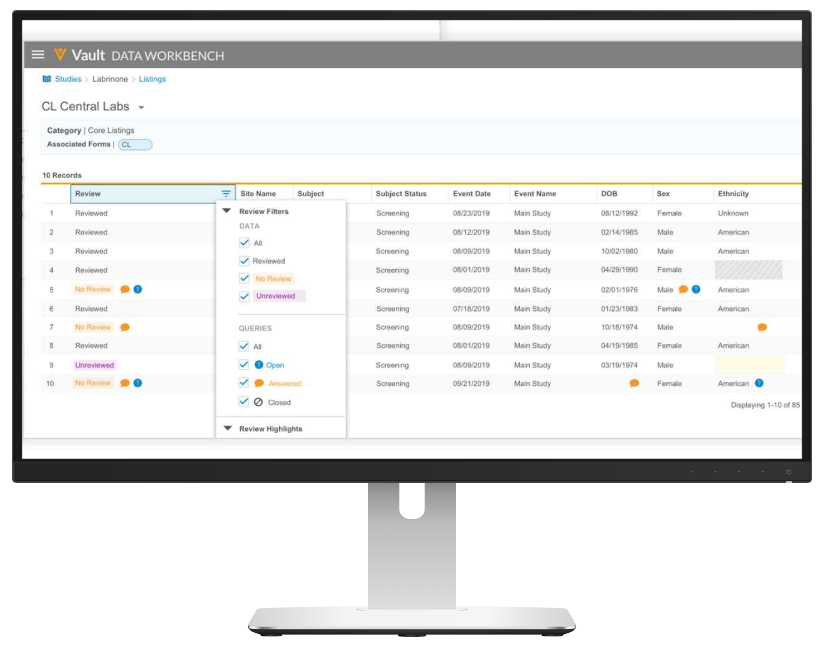
Veeva Vault
- Well-known and very modern system
- Mature EDC system and integrated with a comprehensive clinical suite
- Easily configured for complex, multi-arm adaptive trials
- Integrates easily with well-known Veeva CTMS
Viedoc
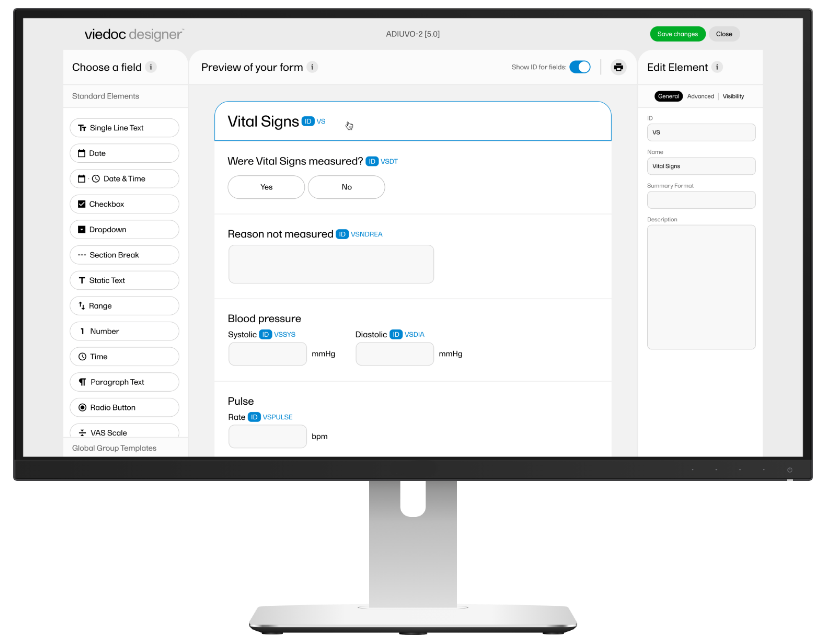
- Well-known in the market
- End-user friendly
- Well suited for decentralized trials due to integrated eConsent module and possibility of tele-visits
- Strong reporting functionality

„For over 30 years, METRONOMIA has been a great home for our employees and, equally as important, for our customers’ data. Try us – you won’t regret it!”












